Abstract
Research Article
Differentiation of bone marrow cells in arthritic mice with decreased complement activity
Petya Ganova and Nina Ivanovska*
Published: 31 December, 2018 | Volume 2 - Issue 1 | Pages: 028-038
There is evidence that complement components induce cell migration in mesenchymal stem cells and regulate cytokine production in osteoblastic cells thus playing a regulatory role in normal bone formation. The aim of the present study was to investigate the involvement of complement system in the differentiation of bone marrow cells in complement-depleted model of rheumatoid arthritis (RA). Arthritis was induced by intraarticular injection of zymosan in cobra venom factor (CVF)-treated mice depleted of functional complement. The expression of different markers by bone marrow [1], on fibroblasts (CD29), mesenchymal cells (CD105), dendritic cells (CD14, CD86), osteoclasts (CD265), cells expressing Dectin1 (CD369) and megakaryocytes (CD62P) was determined by flowcytometry. The lack of functional complement activity at the point of arthritis initiation (day 3) lead to an increase of fibroblast and megakaryocyte populations, to a decrease of mature and dectin1 positive populations, while the number of mesenchymal cells was not changed, all compared to arthritic mice. Immunohistochemical staining showed that low complement activity diminished arthritis-induced generation of megakaryocytes and platelets in BM. Chronic inflammation during erosive conditions such as rheumatoid arthritis, leads to dysregulated differentiation and prolifеration of bone cells, inflammation of synovial membrane and bone marrow, and degradation of cartilage and bone. Present results point that the lack of functional complement changed the ratio between different cell populations that can be used for determining the development and stage of rheumatoid arthritis and can help finding of new therapeutic approaches.
Read Full Article HTML DOI: 10.29328/journal.icci.1001006 Cite this Article Read Full Article PDF
Keywords:
Zymosan-induced arthritis; Dendritic cells; Mesnchymal cells; Fibroblasts; Megakaryocytes
References
- Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006; 169: 987-998. Ref.:.: https://goo.gl/Axuf7P
- Okroj M, Heinegård D, Holmdahl R, Blom AM. Rheumatoid arthritis and the complement system. Ann Med. 2007; 39: 517-530. Ref.: https://goo.gl/T4hFxH
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010; 11: 785-797. Ref.: https://goo.gl/L9XmUX
- Konttinen YT, Ceponis A, Meri S, Vuorikoski A, Kortekangas P, et al. Complement in acute and chronic arthritides: assessment of C3c, C9, and protectin (CD59) in synovial membrane. Ann Rheum Dis. 1996; 55: 888-894. Ref.: https://goo.gl/QcXZaA
- Neumann E, Barnum SR, Tarner IH, Echols J, Fleck M, et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2002; 46: 934-945. Ref.: https://goo.gl/VzNUpi
- O'Gradaigh D, Compston JE. T-cell involvement in osteoclast biology: implications for rheumatoid bone erosion. Rheumatology (Oxford). 2004; 43: 122-130. Ref.: https://goo.gl/pe25MS
- Sato T, Abe E, Jin CH, Hong MH, Katagiri T, et al. The biological roles of the third component of complement in osteoclast formation. Endocrinology. 1993; 133: 397-404. Ref.: https://goo.gl/adpH2m
- Wang X, Wang Y, Gou W, Lu Q, Peng J, et al. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013; 37: 2491-2498. Ref.: https://goo.gl/sy5tJ5
- Farini A, Sitzia C1, Erratico S1, Meregalli M1, Torrente Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int. 2014; 2014: 306573. Ref.: https://goo.gl/uvRvMg
- Kristjánsson B, Honsawek S. Mesenchymal stem cells for cartilage regeneration in osteoarthritis. World J Orthop. 2017; 8: 674-680. Ref.: https://goo.gl/RVv2Nz
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications, FASEB J. 2003; 17: 984-992. Ref.: https://goo.gl/UkUM2y
- Lutzky V, Hannawi S, Thomas R. Cells of the synovium in rheumatoid arthritis. Dendritic cells. Arthritis Res Ther. 2007; 9: 219. Ref.: https://goo.gl/ung6ed
- Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999; 189: 611-614. Ref.: https://goo.gl/7Sfk6N
- Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L. Targeting antigens to dendritic cell receptors for vaccine development. J Drug Deliv. 2013; 2013: 869718 Ref.: https://goo.gl/B5kUTb
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007; 8: 630-638. Ref.: https://goo.gl/jnmsnS
- Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S,, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008; 205: 1447-1461. Ref.: https://goo.gl/JZgKad
- Nakeff A, Maat B. Separation of megakaryocytes from mouse bone marrow by velocity sedimentation. Blood. 1974; 43: 591-595. Ref.: https://goo.gl/8WZ9Qp
- Zou Z, Schmaier AA, Cheng L, Mericko P, Dickeson SK, et al. Negative regulation of activated alpha-2 integrins during thrombopoiesis. Blood. 2009; 113: 6428-6439. Ref.: https://goo.gl/AQkrCF
- Arbesu I, Bucsaiova M, Fischer MB, Mannhalter C. Platelet-borne complement proteins and their role in platelet-bacteria interactions. J Thromb Haemost. 2016; 14: 2241-2252. Ref.: https://goo.gl/BZyCPZ
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000; 289: 1504-1508. Ref.: https://goo.gl/o1FPZg
- Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013; 92: 860-867. Ref.: https://goo.gl/rFcmvG
- Teitelbaum SL. The osteoclast and its unique cytoskeleton. Ann N Y Acad Sci. 2011; 1240: 14-17. Ref.: https://goo.gl/dDERHq
- Belenska-Todorova L, Gyurkovska V, Ivanovska N. How complement activation influences the development of chronic synovitis in a mouse model of rheumatoid arthritis. Scand J Rheumatol. 2016; 45: 13-22. Ref.: https://goo.gl/tjJ7us
- Ganova P, Gyurkovska V, Belenska-Todorova L, Ivanovska N. Functional complement activity is decisive for the development of chronic synovitis, osteophyte formation and processes of cell senescence in zymosan-induced arthritis. Immunol Lett. 2017; 190: 213-220. Ref.: https://goo.gl/6jwmK1
- Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, et al. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001; 22: 199-204. Ref.: https://goo.gl/w2rV1j
- Müller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005; 1: 102-110. Ref.: https://goo.gl/Nv5BMj
- Arend WP, Mehta G, Antonioli AH, Takahashi M, Takahashi K, et al. Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. J Immunol. 2013; 190: 6423-6433. Ref.: https://goo.gl/7B9V1x
- Guc D, Gulati P, Lemercier C, Lappin D, Birnie GD, et al. Expression of the components and regulatory proteins of the alternative complement pathway and the membrane attack complex in normal and diseased synovium. Rheumatol Int. 1993; 13: 139-146. Ref.: https://goo.gl/nwLmWj
- Lefèvre S, Knedla A, Tennie C, Kampmann A, Wunrau C, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009; 15: 1414-1420. Ref.: https://goo.gl/ukVcP8
- Leung BP, Conacher M, Hunter D, McInnes IB, Liew FY, et al. A novel dendritic cell-induced model of erosive inflammatory arthritis: distinct roles for dendritic cells in T cell activation and induction of local inflammation. J Immunol. 2002; 169: 7071-7077. Ref.: https://goo.gl/ukyXSt
- Thomas R, Davis LS, Lipsky PE Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol. 1994; 152: 2613-2623. Ref.: https://goo.gl/2E4Wfp
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999; 13: 2412-2424. Ref.: https://goo.gl/M5AoWD
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999; 397: 315-323. Ref.: https://goo.gl/VgBFef
- Lubberts E, Koenders MI, van den Berg WB The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005; 7: 29-37. Ref.: https://goo.gl/EXBJVk
- Wysoczynski M, Kucia M, Ratajczak J, Ratajczak MZ. Cleavage fragments of the third complement component (C3) enhance stromal derived factor-1 (SDF-1)-mediated platelet production during reactive post bleeding thrombocytosis. Leukemia. 2007; 21: 973-982. https://goo.gl/U1ZLia
- Ciovacco WA, Cheng YH, Horowitz MC, Kacena MA. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J Cell Biochem. 2010; 109: 774-781. Ref.: https://goo.gl/j58HZn
- Underhill DM. Macrophage recognition of zymosan particles. J Endotoxin Res. 2003; 9: 176-180. Ref.: https://goo.gl/Qof2oK
- Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J Immunol. 2003; 170: 5075-5081. Ref.: https://goo.gl/CRn9Es
Figures:
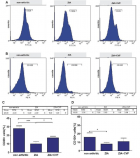
Figure 1

Figure 2

Figure 3
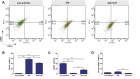
Figure 4

Figure 5

Figure 6
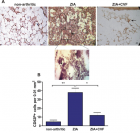
Figure 7
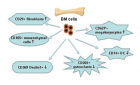
Figure 8
Figure 9
Similar Articles
-
Dendritic cells and TNF-Related apoptosis inducing ligand (TRAIL) represent new possibilities for sepsis treatmentPetya Ganova,Lyudmila Belenska-Todorova,Nina Ivanovska*. Dendritic cells and TNF-Related apoptosis inducing ligand (TRAIL) represent new possibilities for sepsis treatment. . 2017 doi: 10.29328/journal.hcci.1001001; 1: 001-004
-
Differentiation of bone marrow cells in arthritic mice with decreased complement activityPetya Ganova,Nina Ivanovska*. Differentiation of bone marrow cells in arthritic mice with decreased complement activity. . 2018 doi: 10.29328/journal.icci.1001006; 2: 028-038
Recently Viewed
-
Success, Survival and Prognostic Factors in Implant Prosthesis: Experimental StudyEpifania Ettore*, Pietrantonio Maria, Christian Nunziata, Ausiello Pietro. Success, Survival and Prognostic Factors in Implant Prosthesis: Experimental Study. J Oral Health Craniofac Sci. 2023: doi: 10.29328/journal.johcs.1001045; 8: 024-028
-
Agriculture High-Quality Development and NutritionZhongsheng Guo*. Agriculture High-Quality Development and Nutrition. Arch Food Nutr Sci. 2024: doi: 10.29328/journal.afns.1001060; 8: 038-040
-
A Low-cost High-throughput Targeted Sequencing for the Accurate Detection of Respiratory Tract PathogenChangyan Ju, Chengbosen Zhou, Zhezhi Deng, Jingwei Gao, Weizhao Jiang, Hanbing Zeng, Haiwei Huang, Yongxiang Duan, David X Deng*. A Low-cost High-throughput Targeted Sequencing for the Accurate Detection of Respiratory Tract Pathogen. Int J Clin Virol. 2024: doi: 10.29328/journal.ijcv.1001056; 8: 001-007
-
A Comparative Study of Metoprolol and Amlodipine on Mortality, Disability and Complication in Acute StrokeJayantee Kalita*,Dhiraj Kumar,Nagendra B Gutti,Sandeep K Gupta,Anadi Mishra,Vivek Singh. A Comparative Study of Metoprolol and Amlodipine on Mortality, Disability and Complication in Acute Stroke. J Neurosci Neurol Disord. 2025: doi: 10.29328/journal.jnnd.1001108; 9: 039-045
-
Development of qualitative GC MS method for simultaneous identification of PM-CCM a modified illicit drugs preparation and its modern-day application in drug-facilitated crimesBhagat Singh*,Satish R Nailkar,Chetansen A Bhadkambekar,Suneel Prajapati,Sukhminder Kaur. Development of qualitative GC MS method for simultaneous identification of PM-CCM a modified illicit drugs preparation and its modern-day application in drug-facilitated crimes. J Forensic Sci Res. 2023: doi: 10.29328/journal.jfsr.1001043; 7: 004-010
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."